- Important Safety Information
- Indications
- Prescribing Information
- Patient Information
- For US Health Care Professionals
- Patient Site
SYMBICORT 160/4.5 for the maintenance treatment of COPD
The majority of patients’ FEV1 improvement occurred at 5 minutes in COPD1-3

In a serial spirometry subset of patients taking SYMBICORT 160/4.5* (n=121) in the SUN Study, 67% of 1-hour postdose FEV1 improvement occurred at 5 minutes on day of randomization, 83% at month 6, and 84% at end of treatment1-3
Sustained improvement in lung function was demonstrated in COPD in a 12-month efficacy and safety study1,2
*Administered as 2 inhalations twice daily.
SYMBICORT is NOT a rescue medication and does NOT replace fast-acting inhalers to treat acute symptoms
The most common adverse reactions ≥3% reported in COPD lung function clinical trials included nasopharyngitis, oral candidiasis, bronchitis, sinusitis, and upper respiratory tract infection.
STUDY DESIGN & COMPARATOR ARMS: Lung Function Study
Study 2 (SUN): A 12-month, randomized, double-blind, double-dummy, placebo-controlled, parallel-group, multicenter study of 1964 patients with COPD compared SYMBICORT pMDI 160/4.5 mcg (n=494), SYMBICORT pMDI 80/4.5 mcg (n=494), formoterol 4.5 mcg (n=495), and placebo (n=481), each administered as 2 inhalations twice daily. Subjects were current or ex-smokers with a smoking history of ≥10 pack-years, aged ≥40 years with a clinical diagnosis of COPD and symptoms for >2 years.
The study included a 2-week run-in period followed by a 12-month treatment period. This study was designed to assess change from baseline to the average over the randomized treatment period in predose FEV1 and in 1-hour postdose FEV1. The prespecified primary comparisons for predose FEV1 were vs placebo and formoterol and the primary comparison for 1-hour postdose was vs placebo.
COMPARATOR ARMS
Mean improvement in 1-hour postdose FEV1 (mL/%) over 12 months (serial spirometry subset):
Day of randomization
SYMBICORT 160/4.5 mcg: 240 mL/26%
Formoterol 4.5 mcg: 180 mL/20%
Placebo: 40 mL/5%
6 months
SYMBICORT 160/4.5 mcg: 270 mL/28%
Formoterol 4.5 mcg: 200 mL/23%
Placebo: 60 mL/7%
End of month 12 (LOCF)
SYMBICORT 160/4.5 mcg: 240 mL/26%
Formoterol 4.5 mcg: 170 mL/19%
Placebo: 30 mL/5%
SYMBICORT 160/4.5 mcg* (n=121)
Formoterol 4.5 mcg* (n=124)
Placebo* (n=125)
*Administered as 2 inhalations twice daily.
SYMBICORT 160/4.5 for reducing COPD exacerbations
In a 12-month exacerbation clinical trial (Study 4), SYMBICORT 160/4.5 significantly reduced the annual rate of moderate/severe COPD exacerbations vs formoterol3,4
Annual rate estimate:
1.05, formoterol 4.5 mcg* (n=403)

P<.0001 vs formoterol4
Estimate Rate Ratio=0.65;
95% CI: 0.53, 0.80
Annual rate estimate:
0.68, SYMBICORT 160/4.5 mcg* (n=404)
In a second exacerbation clinical trial of 6-month duration (Study 3), SYMBICORT 160/4.5 significantly reduced the annual rate of moderate/severe COPD exacerbations by 26% vs formoterol (Estimate Rate Ratio=0.74; 95% CI: 0.61, 0.91; P=.004)3,4
Annual rate estimate was 0.94 for SYMBICORT 160/4.5 mcg* (n=606) vs 1.27 for formoterol 4.5 mcg* (n=613)
*Administered as 2 inhalations twice daily.
In Study 3, COPD exacerbations were defined as worsening of ≥2 major symptoms (dyspnea, sputum volume, sputum color/purulence) or worsening of any 1 major symptom together with ≥1 of the minor symptoms (sore throat, colds [nasal discharge and/or nasal congestion], fever without other cause, increased cough or increased wheeze) for ≥2 consecutive days. COPD exacerbation severity was classified as moderate if symptoms required systemic corticosteroid (≥3 days) and/or antibiotic treatment, and severe if symptoms required hospitalization.
In Study 4, COPD exacerbations were defined as worsening of COPD that required treatment with a course of oral steroids and/or hospitalization.
The safety findings from the two exacerbation clinical trials were consistent with the lung function studies.
STUDY DESIGN: Exacerbation Studies
Study 3 (RISE): A 6-month, Phase IIIB, randomized, double-blind, double-dummy, parallel-group, multicenter study of 1219 patients with COPD compared SYMBICORT pMDI 160/4.5 mcg (n=606) with formoterol 4.5 mcg (n=613), each administered as 2 inhalations twice daily. Subjects were current or ex-smokers with a smoking history of ≥10 pack-years, aged ≥40 years with a clinical diagnosis of COPD, COPD symptoms for >1 year, and a history of ≥1 moderate or severe COPD exacerbation in the previous year requiring treatment with systemic corticosteroids or hospitalization. The study included a 4-week run-in period, a 26-week randomized treatment period, and telephone follow-up 2 weeks after end of study completion. This study was designed to assess the annual rate of moderate and severe COPD exacerbations for SYMBICORT vs formoterol.
Study 4: A 12-month, Phase IIIB, randomized, double-blind, double-dummy, parallel-group, multicenter study of 811 patients with COPD compared SYMBICORT pMDI 160/4.5 mcg (n=407) with formoterol 4.5 mcg (n=404), each administered as 2 inhalations twice daily. Subjects were current or ex-smokers with a smoking history of ≥10 pack-years, aged ≥40 years with a clinical diagnosis of COPD, COPD symptoms for >2 years, and a history of ≥1 COPD exacerbation in the previous year treated with a course of systemic corticosteroids and/or antibiotics. The study included a 2-week run-in period, a 12-month randomized treatment period, and telephone follow-up 2 weeks after end of study completion. This study was designed to assess the annual rate of COPD exacerbations for SYMBICORT vs formoterol.
SYMBICORT 160/4.5 for the maintenance treatment of COPD
SYMBICORT is NOT a rescue medication and does NOT replace fast-acting inhalers to treat acute symptoms
SUN: A 12-month efficacy and safety study
*Administered as 2 inhalations twice daily.
†Baseline is defined as the predose FEV1 value on the day of randomization.
‡Month 12, last observation carried forward.
STUDY DESIGN & COMPARATOR ARMS
Study 2 (SUN): A 12-month, randomized, double-blind, double-dummy, placebo-controlled, parallel-group, multicenter study of 1964 patients with COPD compared SYMBICORT pMDI 160/4.5 mcg (n=494), SYMBICORT pMDI 80/4.5 mcg (n=494), formoterol 4.5 mcg (n=495), and placebo (n=481), each administered as 2 inhalations twice daily. Subjects were current or ex-smokers with a smoking history of ≥10 pack-years, aged ≥40 years with a clinical diagnosis of COPD and symptoms for >2 years.
The study included a 2-week run-in period followed by a 12-month treatment period. This study was designed to assess change from baseline to the average over the randomized treatment period in predose FEV1 and in 1-hour postdose FEV1. The prespecified primary comparisons for predose FEV1 were vs placebo and formoterol and the primary comparison for 1-hour postdose was vs placebo.
COMPARATOR ARMS
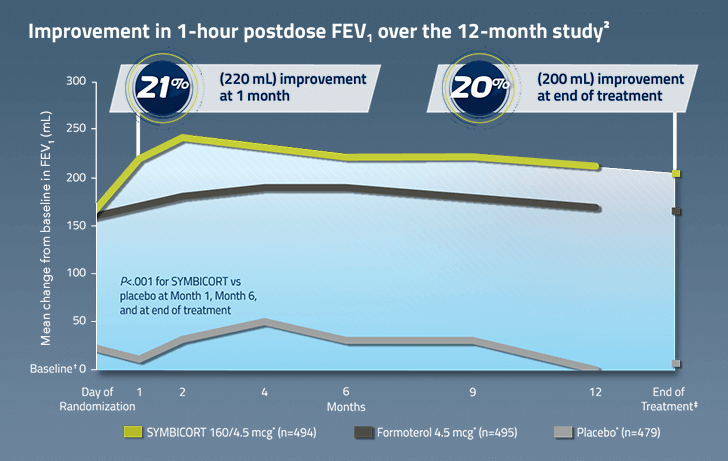
Mean improvement in 1-hour postdose FEV1 (mL/%) over 12 months:
Month 1
SYMBICORT 160/4.5 mcg: 220 mL/21%
Formoterol 4.5 mcg: 170 mL/17%
Placebo: 10 mL/1%
Month 2
SYMBICORT 160/4.5 mcg: 240 mL/23%
Formoterol 4.5 mcg: 180 mL/17%
Placebo: 30 mL/3%
Month 4
SYMBICORT 160/4.5 mcg: 230 mL/22%
Formoterol 4.5 mcg: 190 mL/18%
Placebo: 50 mL/5%
Month 6
SYMBICORT 160/4.5 mcg: 220 mL/21%
Formoterol 4.5 mcg: 190 mL/18%
Placebo: 30 mL/3%
Month 9
SYMBICORT 160/4.5 mcg: 220 mL/21%
Formoterol 4.5 mcg: 180 mL/17%
Placebo: 30 mL/3%
Month 12
SYMBICORT 160/4.5 mcg: 210 mL/20%
Formoterol 4.5 mcg: 170 mL/16%
Placebo: 0 mL/0%
End of treatment
SYMBICORT 160/4.5 mcg: 200 mL/20%
Formoterol 4.5 mcg: 170 mL/17%
Placebo: 10 mL/1%
SYMBICORT 160/4.5 mcg* (n=494)
Formoterol 4.5 mcg* (n=495)
Placebo* (n=479)
*Administered as 2 inhalations twice daily.
SYMBICORT 160/4.5 for the maintenance treatment of COPD
SYMBICORT is NOT a rescue medication and does NOT replace fast-acting inhalers to treat acute symptoms
SUN: A 12-month efficacy and safety study
6-Month Study (SHINE) Results
The most common adverse reactions ≥3% reported in COPD lung function clinical trials included nasopharyngitis, oral candidiasis, bronchitis, sinusitis, and upper respiratory tract infection
*Administered as 2 inhalations twice daily.
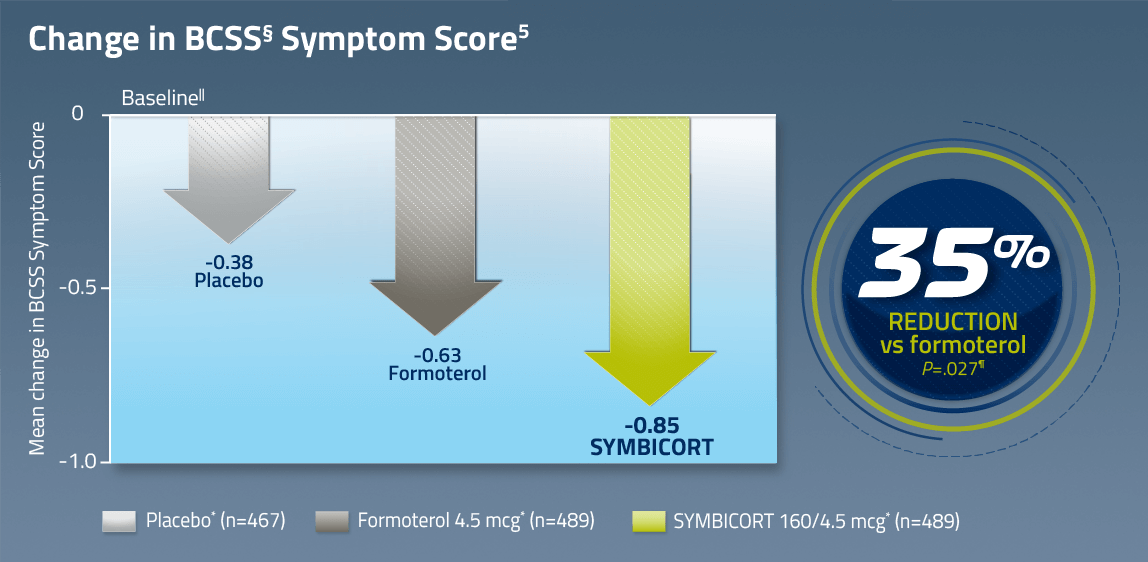
§The Breathlessness, Cough, and Sputum Scale (BCSS) was a secondary endpoint measured in diary cards every day before the evening dose. Patients were asked to evaluate each symptom/item on a Likert-type scale ranging from 0 to 4, with higher scores indicating a more severe manifestation of the symptom. A total symptom score is expressed as the sum of 3 item scores, with a range from 0 to 12.
||Baseline is defined as the mean of all values obtained during the last 10 days of the run-in period. Mean BCSS baseline values were 5.35 for SYMBICORT 160/4.5, 5.39 for formoterol 4.5, and 5.34 for placebo.
¶P values based on treatment comparison of absolute mean change from baseline for SYMBICORT vs formoterol and placebo.
STUDY DESIGN
Study 1 (SHINE): A 6-month, randomized, double-blind, double-dummy, placebo-controlled, parallel-group, multicenter study of 1704 patients with COPD compared SYMBICORT pressurized metered-dose inhaler (pMDI) 160/4.5 mcg (n=277), SYMBICORT pMDI 80/4.5 mcg (n=281), budesonide 160 mcg (n=275), formoterol 4.5 mcg (n=284), the free combination of budesonide 160 mcg plus formoterol 4.5 mcg (n=287), and placebo (n=300), each administered as 2 inhalations twice daily. Subjects were current or ex-smokers with a smoking history of ≥10 pack-years, aged ≥40 years with a clinical diagnosis of COPD and symptoms for >2 years.
The study included a 2-week run-in period followed by a 6-month treatment period. This study was designed to assess change from baseline to the average over the randomized treatment period in predose FEV1 and in 1-hour postdose FEV1. The prespecified primary comparison for predose FEV1 was vs formoterol and for 1-hour postdose was vs budesonide.
Study 2 (SUN): A 12-month, randomized, double-blind, double-dummy, placebo-controlled, parallel-group, multicenter study of 1964 patients with COPD compared SYMBICORT pMDI 160/4.5 mcg (n=494), SYMBICORT pMDI 80/4.5 mcg (n=494), formoterol 4.5 mcg (n=495), and placebo (n=481), each administered as 2 inhalations twice daily. Subjects were current or ex-smokers with a smoking history of ≥10 pack-years, aged ≥40 years with a clinical diagnosis of COPD and symptoms for >2 years.
The study included a 2-week run-in period followed by a 12-month treatment period. This study was designed to assess change from baseline to the average over the randomized treatment period in predose FEV1 and in 1-hour postdose FEV1. The prespecified primary comparisons for predose FEV1 were vs placebo and formoterol and the primary comparison for 1-hour postdose was vs placebo.
SYMBICORT 160/4.5 for the maintenance treatment of COPD
SYMBICORT is NOT a rescue medication and does NOT replace fast-acting inhalers to treat acute symptoms
SUN: A 12-month efficacy and safety study
6-Month Study (SHINE) Results
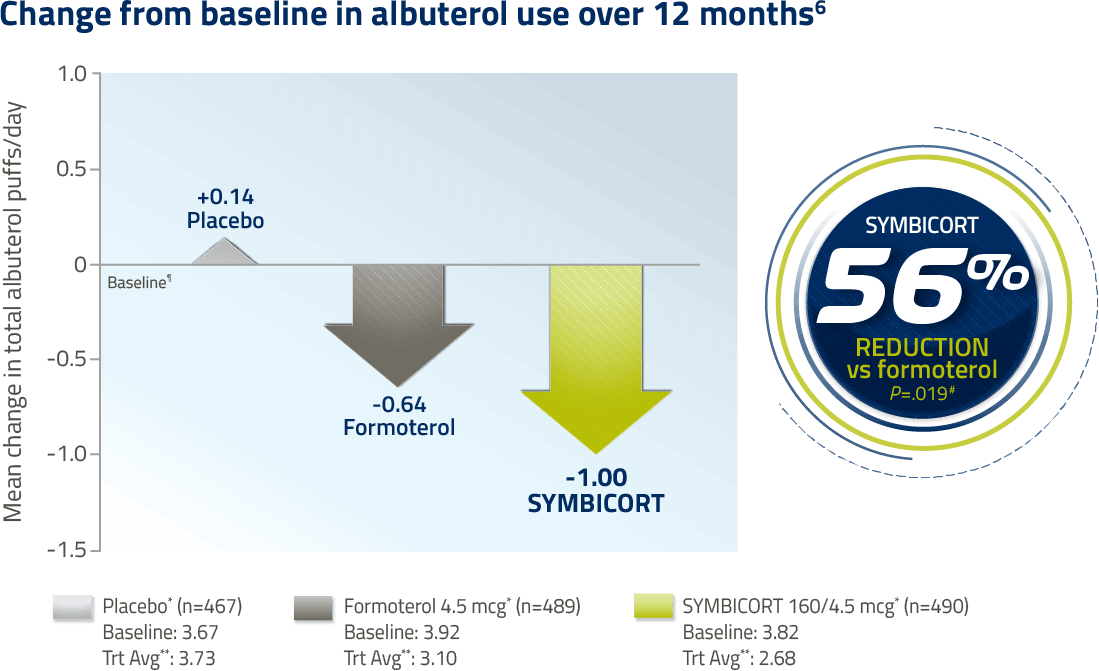
In a 6-month study, the reduction in rescue medication use for SYMBICORT 160/4.5 vs formoterol was not significant (25% decrease, P=.280). Rescue medication use was a secondary endpoint, with the primary comparison being SYMBICORT vs placebo (P<.001).
The most common adverse reactions ≥3% reported in COPD lung function clinical trials included nasopharyngitis, oral candidiasis, bronchitis, sinusitis, and upper respiratory tract infection
*Administered as 2 inhalations twice daily.
¶Baseline is defined as the mean of all values obtained during the last 10 days of the run-in period.
#P values based on treatment comparison of absolute mean change from baseline for SYMBICORT vs formoterol and placebo.
**Treatment Average (Trt Avg) is defined as the mean of all values obtained during the double-blind treatment period.
STUDY DESIGN
Study 1 (SHINE): A 6-month, randomized, double-blind, double-dummy, placebo-controlled, parallel-group, multicenter study of 1704 patients with COPD compared SYMBICORT pressurized metered-dose inhaler (pMDI) 160/4.5 mcg (n=277), SYMBICORT pMDI 80/4.5 mcg (n=281), budesonide 160 mcg (n=275), formoterol 4.5 mcg (n=284), the free combination of budesonide 160 mcg plus formoterol 4.5 mcg (n=287), and placebo (n=300), each administered as 2 inhalations twice daily. Subjects were current or ex-smokers with a smoking history of ≥10 pack-years, aged ≥40 years with a clinical diagnosis of COPD and symptoms for >2 years.
The study included a 2-week run-in period followed by a 6-month treatment period. This study was designed to assess change from baseline to the average over the randomized treatment period in predose FEV1 and in 1-hour postdose FEV1. The prespecified primary comparison for predose FEV1 was vs formoterol and for 1-hour postdose was vs budesonide.
Study 2 (SUN): A 12-month, randomized, double-blind, double-dummy, placebo-controlled, parallel-group, multicenter study of 1964 patients with COPD compared SYMBICORT pMDI 160/4.5 mcg (n=494), SYMBICORT pMDI 80/4.5 mcg (n=494), formoterol 4.5 mcg (n=495), and placebo (n=481), each administered as 2 inhalations twice daily. Subjects were current or ex-smokers with a smoking history of ≥10 pack-years, aged ≥40 years with a clinical diagnosis of COPD and symptoms for >2 years.
The study included a 2-week run-in period followed by a 12-month treatment period. This study was designed to assess change from baseline to the average over the randomized treatment period in predose FEV1 and in 1-hour postdose FEV1. The prespecified primary comparisons for predose FEV1 were vs placebo and formoterol and the primary comparison for 1-hour postdose was vs placebo.
SYMBICORT 160/4.5 for the maintenance treatment of COPD and for reducing COPD exacerbations.
Incidence of Pneumonia
Physician should remain vigilant for the possible development of pneumonia in patients with COPD. There was a higher incidence of lung infections other than pneumonia in patients receiving SYMBICORT vs placebo.3
Incidence of pneumonia was not greater in the SYMBICORT 160/4.5 mcg group compared with placebo in the pooled safety data from the lung function studies (SUN and SHINE)7
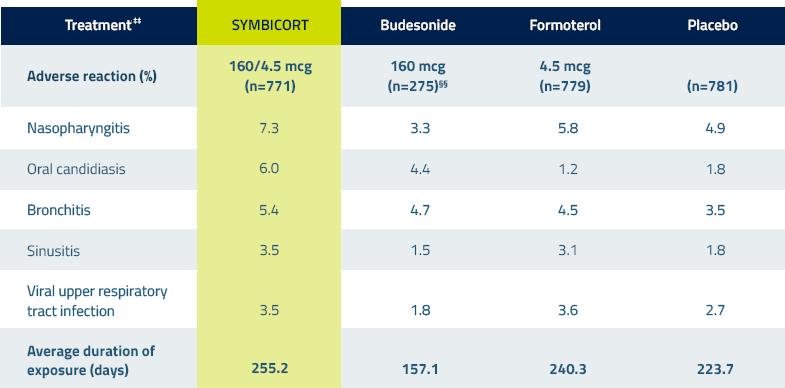
Adverse reactions in COPD lung function studies 3††‡‡
Adverse reactions occurring in the COPD lung function clinical studies at an incidence of ≥3% and more commonly than placebo in the SYMBICORT group3
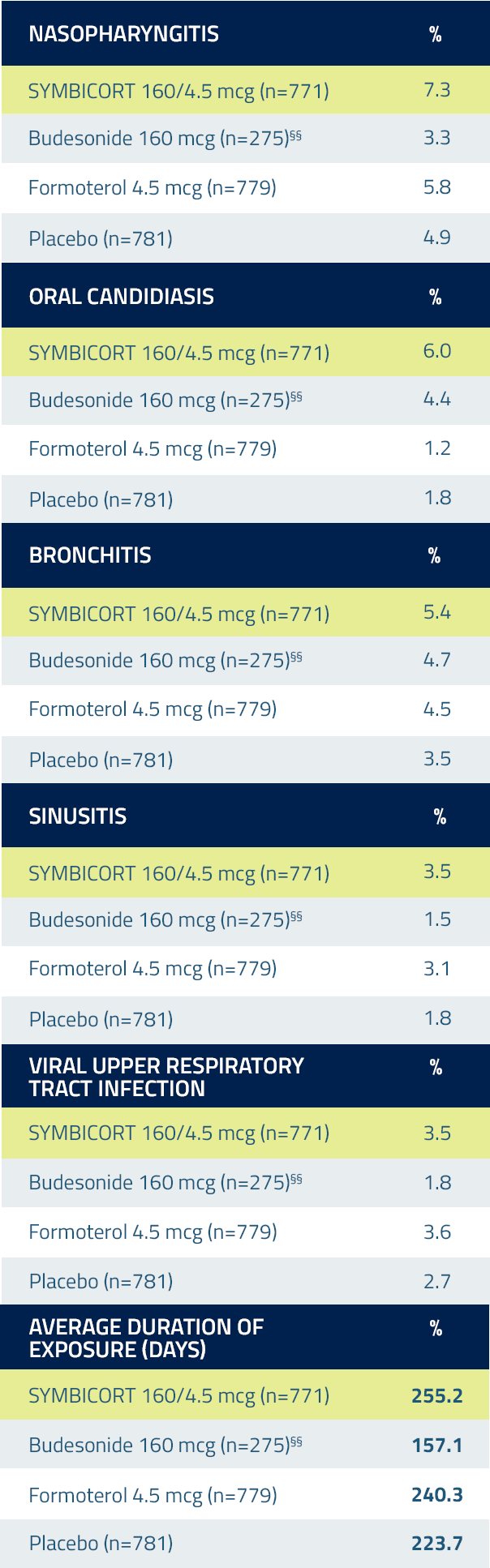
The safety findings from the 2 exacerbation clinical trials were consistent with the lung function studies.
††Combined data from 2 pivotal clinical lung function studies of patients with COPD taking SYMBICORT.
‡‡All treatments were administered as 2 inhalations twice daily.
§§Because the SUN Study did not include a budesonide arm, there are fewer patients in this treatment group.
Please see full Prescribing Information ![]() , including Patient Information.
, including Patient Information.