- Important Safety Information
- Indications
- Prescribing Information
- Patient Information
- For US Health Care Professionals
- Patient Site
See Asthma Safety Profile in asthma patients 12 years and older.
The majority of patients’ FEV1 improvement occurred at 15 minutes in asthma1-3
In patients ≥12 years of age with asthma taking SYMBICORT 160/4.5* (n=124) in Study 1, 79% of 2-hour postdose FEV1 improvement occurred at 15 minutes on day of randomization, 89% at week 2, and 90% at end of treatment1-3
Sustained improvement in lung function was demonstrated in asthma patients ≥12 years of age in a 12-week efficacy and safety study2,3
Patients saw sustained effect over 12 weeks2-3
Study 1: A 12-week efficacy and safety study of patients with moderate to severe asthma
SYMBICORT 160/4.5* significantly improved predose FEV1† (P <.05 vs budesonide, formoterol, and placebo) averaged over the course of the study, and also improved 12-hour average postdose FEV1† (P <.001 vs budesonide, formoterol, and placebo at week 2), coprimary endpoints3; 2-hour postdose FEV1† over 12 weeks was a secondary endpoint2
*Administered as 2 inhalations twice daily.
†As compared to baseline; baseline defined as the predose FEV1 value on day of randomization.
The most common adverse reactions ≥3% reported in asthma clinical trials included nasopharyngitis, headache, upper respiratory tract infection, pharyngolaryngeal pain, sinusitis, influenza, back pain, nasal congestion, stomach discomfort, vomiting, and oral candidiasis.
STUDY DESIGN & COMPARATOR ARMS
Study 1: A 12-week, double-blind, placebo-controlled study comparing SYMBICORT 160/4.5 mcg, budesonide 160 mcg, formoterol 4.5 mcg, the free combination of budesonide 160 mcg plus formoterol 4.5 mcg in separate inhalers, and placebo, each administered as 2 inhalations twice daily. A total of 596 patients (124 randomized to receive SYMBICORT) ≥12 years of age were evaluated. The study included a 2-week run-in period with budesonide 80 mcg, 2 inhalations twice daily. Most patients had moderate to severe asthma and were using moderate to high doses of inhaled corticosteroids (ICSs) prior to study entry. This study was designed to assess 2 primary endpoints. The first was predose FEV1 averaged over 12 weeks, and the second was 12-hour average postdose FEV1 at Week 2. Secondary efficacy variables included daytime and nighttime asthma symptom scores and daily rescue medication use (both recorded by patients in the electronic diary).
COMPARATOR ARMS
Mean change in 2-hour postdose FEV1 (mL/%) over 12 weeks:
Day of randomization
SYMBICORT 160/4.5 mcg: 420 mL/20.0%
Budesonide 160 mcg: 100 mL/4.4%
Formoterol 4.5 mcg: 420 mL/19.9%
Budesonide 160 mcg + formoterol 4.5 mcg: 410 mL/19.4%
Placebo: 90 mL/4.4%
2 weeks
SYMBICORT 160/4.5 mcg: 380 mL/18.6%
Budesonide 160 mcg: 120 mL/5.6%
Formoterol 4.5 mcg: 270 mL/12.8%
Budesonide 160 mcg + formoterol 4.5 mcg: 370 mL/18.0%
Placebo: 10 mL/1.2%
End of treatment
SYMBICORT 160/4.5 mcg: 420 mL/20.2%
Budesonide 160 mcg: 140 mL/6.5%
Formoterol 4.5 mcg: 260 mL/12.3%
Budesonide 160 mcg + formoterol 4.5 mcg: 410 mL/19.5%
Placebo: -10 mL/0.4%
MEAN CHANGE FROM BASELINE* IN ALBUTEROL USE (%)

Study 1: A 12-week efficacy and safety study of patients ≥12 years of age with moderate to severe asthma
SYMBICORT 160/4.5† provided a 70% reduction in albuterol use vs baseline within 1 day of the first dose and a 57% reduction over 12 weeks1,4
Rescue use within 1 day of first dose of SYMBICORT (n=120): Baseline*: 2.12; Treatment‡: 0.911,4
Rescue use over 12 weeks with SYMBICORT (n=121): Baseline*: 2.10; Treatment Average§: 1.091,4
The primary comparison for this secondary endpoint was SYMBICORT vs placebo over 12 weeks (P <.001)4¶
Study 2: A 12-week efficacy and safety study of patients ≥12 years of age with mild to moderate asthma
SYMBICORT 80/4.5† reduced rescue medication use by 51% vs baseline within 1 day of the first dose and 67% over 12 weeks1,4
Study 1: A subset of patients ≥18 years of age with moderate to severe asthma completed a 5-item Onset of Effect Questionnaire
In this subset, a significantly greater percentage of patients taking SYMBICORT 160/4.5† compared with placebo patients felt their medication started working right away at week 1 (69.1% [n=65/94]) versus placebo (23.3% [n=21/90], P <0.001)5,6
Results at the end of treatment (Week 12) remained statistically significant (P <0.001)5,6
SYMBICORT is NOT a rescue medication and does NOT replace fast-acting inhalers to treat acute symptoms
*Baseline is defined as the mean of all values obtained during the run-in period. During run-in, patients received budesonide 80 mcg administered as 2 inhalations twice daily and albuterol as a rescue medication.
†Administered as 2 inhalations twice daily.
‡Treatment is the mean value in puffs/day of albuterol used within 1 day of the first dose of SYMBICORT.
§Treatment Average is defined as the mean of all values obtained during the double-blind treatment period in puffs/day of albuterol.
IIAssessed using the Onset of Effect Questionnaire.
¶P values based on treatment comparison of absolute mean change from baseline for SYMBICORT vs budesonide and placebo.
STUDY DESIGN & COMPARATOR ARMS
Study 1: A 12-week, double-blind, placebo-controlled study comparing SYMBICORT 160/4.5 mcg, budesonide 160 mcg, formoterol 4.5 mcg, the free combination of budesonide 160 mcg plus formoterol 4.5 mcg in separate inhalers, and placebo, each administered as 2 inhalations twice daily. A total of 596 patients (124 randomized to receive SYMBICORT) ≥12 years of age were evaluated. The study included a 2-week run-in period with budesonide 80 mcg, 2 inhalations twice daily. Most patients had moderate to severe asthma and were using moderate to high doses of ICS prior to study entry. This study was designed to assess 2 primary endpoints. The first was predose FEV1 averaged over 12 weeks, and the second was 12-hour average postdose FEV1 at Week 2. Secondary efficacy variables included daytime and nighttime asthma symptom scores, daily rescue medication use (both recorded by patients in the electronic diary), and percent of patients who experienced a predefined asthma event.
Study 2: A 12-week, randomized, multicenter, double-blind, double-dummy, placebo-controlled study comparing SYMBICORT 80/4.5 mcg, budesonide 80 mcg, formoterol 4.5 mcg, each administered as 2 inhalations twice daily. A total of 480 patients (123 randomized to receive SYMBICORT) ≥12 years of age were evaluated. The study included a 2-week run-in period with placebo and rescue albuterol therapy. Most patients had mild to moderate persistent asthma and were using low to moderate doses of ICS either alone or as part of combination therapy prior to study entry. This study was designed to assess 2 primary endpoints. The first was predose FEV1 averaged over 12 weeks, and the second was 12-hour average postdose FEV1 at Week 2. Secondary efficacy variables included daytime and nighttime asthma symptom scores, daily rescue medication use (both recorded by patients in the electronic diary), and percent of patients who experienced a predefined asthma event.
Mean change from baseline in albuterol use within 1 day of the first dose of study treatment
SYMBICORT 160/4.5 mcg: -70% (n=120)
Budesonide 160 mcg: -14% (n=105)
Formoterol 4.5 mcg: -50% (n=117)
Budesonide 160 mcg + formoterol 4.5 mcg: -70% (n=112)
Placebo: -8% (n=122)
Mean change from baseline in albuterol use over 12 weeks
SYMBICORT 160/4.5 mcg: -57% (n=121)
Budesonide 160 mcg: -19% (n=109)
Formoterol 4.5 mcg: -22% (n=119)
Budesonide 160 mcg + formoterol 4.5 mcg: -67% (n=113)
Placebo: 29% (n=124)
Onset of Effect Questionnaire (OEQ): A subset of patients ≥18 years of age was assessed on a weekly basis using the OEQ, administered through an electronic diary to record patients’ responses to 5 statements concerning onset of medication. Responses to OEQ items were analyzed at the end of Week 1 of treatment and at end of treatment (last assessment while on treatment), with the end of Week 1 prespecified as the primary time point to assess patient perception of onset associated with treatment initiation. A responder analysis was conducted for the OEQ, with patient responses of “strongly agree” and “somewhat agree” prespecified as a yes response and responses of “neither agree or disagree,” “somewhat disagree,” and “strongly disagree” prespecified as a no response.
*Patients were considered to have experienced a “predefined asthma event” if any of the following conditions occurred at any time during the study:
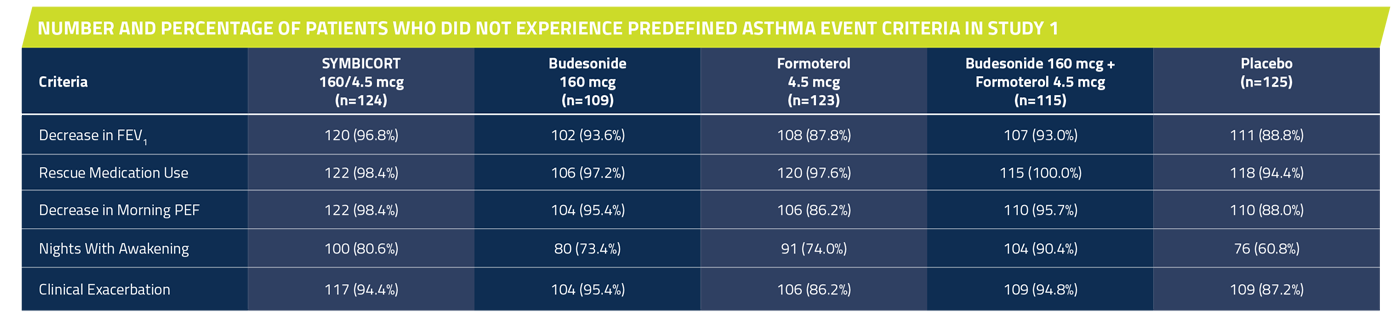
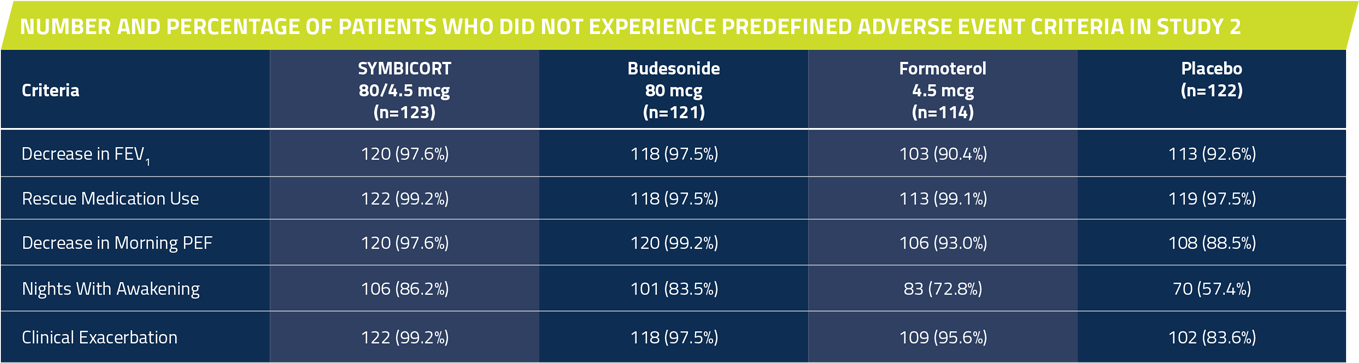
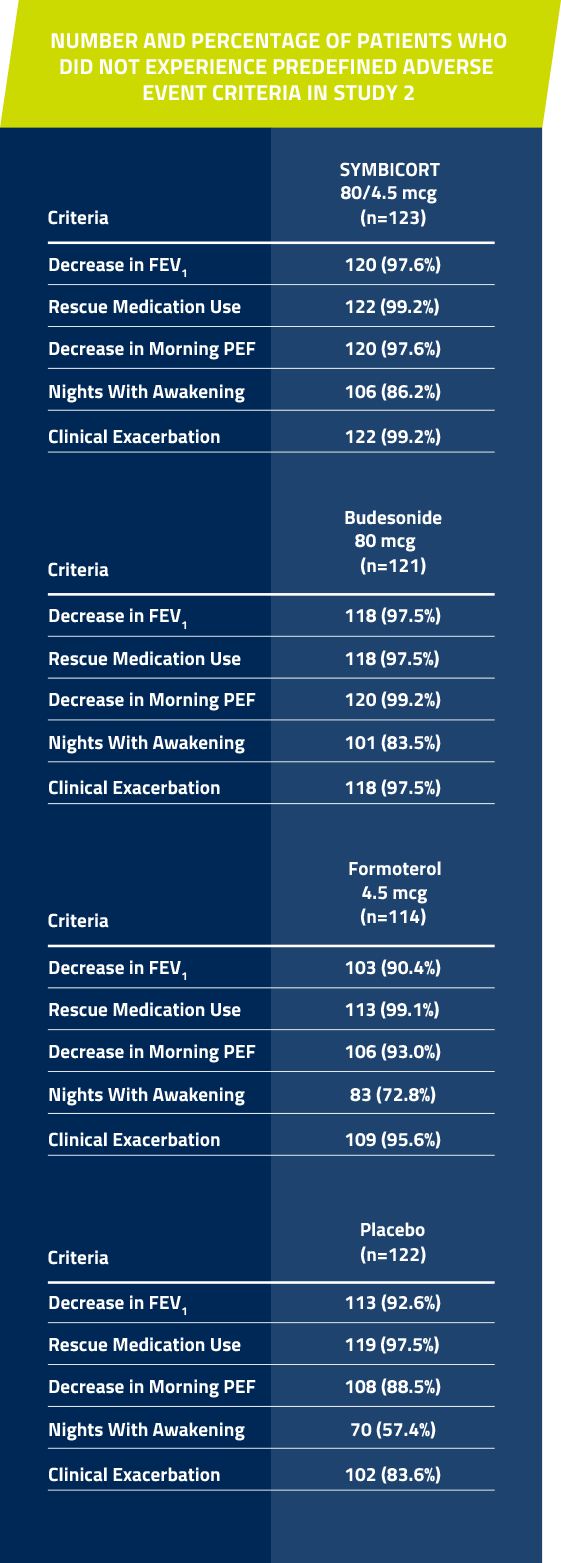
In Studies 1 and 2, significantly more patients taking SYMBICORT* did not have a predefined asthma event† vs placebo over 12 weeks1,7
Individual components are combined for patients with moderate to severe asthma receiving SYMBICORT 160/4.5* in Study 1 (n=124) and for patients with mild to moderate asthma receiving SYMBICORT 80/4.5* in Study 2 (n=123)1,7
National institutes of health defines control in terms of risk and impairment8
For more information, click here
SYMBICORT is NOT a rescue medication and does NOT replace fast-acting inhalers to treat acute symptoms
*Administered as 2 inhalations twice daily.
†Predefined asthma event criteria were a clinically important decrease in FEV1 or PEF, increase in rescue albuterol use, nighttime awakening due to asthma, emergency intervention or hospitalization due to asthma, or requirement for asthma medication not allowed by the protocol.
‡OCS was one of the asthma medications that were not allowed by the protocol.
STUDY DESIGN & COMPARATOR ARMS
Study 1: A 12-week, double-blind, placebo-controlled study comparing SYMBICORT 160/4.5 mcg, budesonide 160 mcg, formoterol 4.5 mcg, the free combination of budesonide 160 mcg plus formoterol 4.5 mcg in separate inhalers, and placebo, each administered as 2 inhalations twice daily. A total of 596 patients (124 randomized to receive SYMBICORT) ≥12 years of age were evaluated. The study included a 2-week run-in period with budesonide 80 mcg, 2 inhalations twice daily. Most patients had moderate to severe asthma and were using moderate to high doses of ICS prior to study entry. This study was designed to assess 2 primary endpoints. The first was predose FEV1 averaged over 12 weeks, and the second was 12-hour average postdose FEV1 at Week 2. Secondary efficacy variables included daytime and nighttime asthma symptom scores, daily rescue medication use (both recorded by patients in the electronic diary), and percent of patients who experienced a predefined asthma event.
COMPARATOR ARMS
Percent of patients who did not have a predefined asthma event*
SYMBICORT 160/4.5 mcg: 70.2% (87/124)
Budesonide 160 mcg: 56% (61/109)
Formoterol 4.5 mcg: 44.7% (55/123)
Budesonide 160 mcg + formoterol 4.5 mcg: 79.1% (91/115)
Placebo: 32.8% (41/125)
The primary comparison was between SYMBICORT and placebo. SYMBICORT 160/4.5 mcg vs placebo (P <0.001).

Study 2: A 12-week, randomized,multicenter, double-blind, double-dummy, placebo-controlled study comparing SYMBICORT 80/4.5 mcg, budesonide 80 mcg, formoterol 4.5 mcg, each administered as 2 inhalations twice daily. A total of 480 patients (123 randomized to receive SYMBICORT) ≥12 years of age were evaluated. The study included a 2-week run-in period with placebo and rescue albuterol therapy. Most patients had mild to moderate persistent asthma and were using low to moderate doses of ICS either alone or as part of combination therapy prior to study entry. This study was designed to assess 2 primary endpoints. The first was predose FEV1 averaged over 12 weeks, and the second was 12-hour average postdose FEV1 at Week 2. Secondary efficacy variables included daytime and nighttime asthma symptom scores, daily rescue medication use (both recorded by patients in the electronic diary), and percent of patients who experienced a predefined asthma event.
COMPARATOR ARMS
Percent of patients who did not have a predefined asthma event*
SYMBICORT 80/4.5 mcg: 81.3% (100/123)
Budesonide 80 mcg: 78.5% (95/121)
Formoterol 4.5 mcg: 57.9% (66/114)
Placebo: 43.4% (53/122)
The primary comparison was between SYMBICORT and placebo. SYMBICORT 80/4.5 mcg vs placebo (P <0.001).
*Patients were considered to have experienced a “predefined asthma event” if any of the following conditions occurred at any time during the study:
SYMBICORT for asthma patients ≥12 years of age uncontrolled on an ICS
SYMBICORT is NOT a rescue medication and does NOT replace fast-acting inhalers to treat acute symptoms.
Adverse reactions in clinical studies1 asthma patients ≥12 years of age
Adverse reactions occurring at an incidence of ≥3% and more commonly than placebo in the SYMBICORT groups1
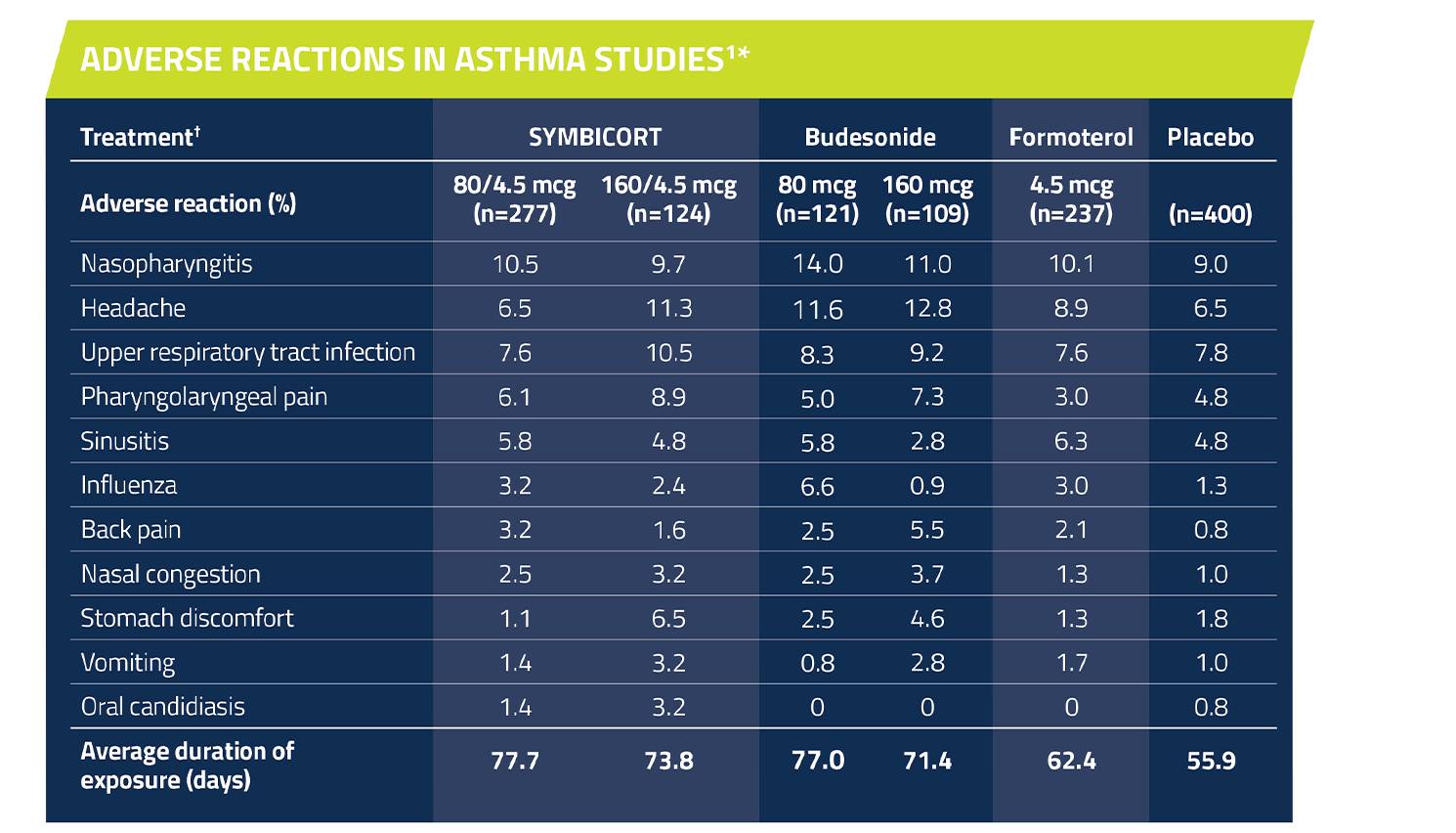
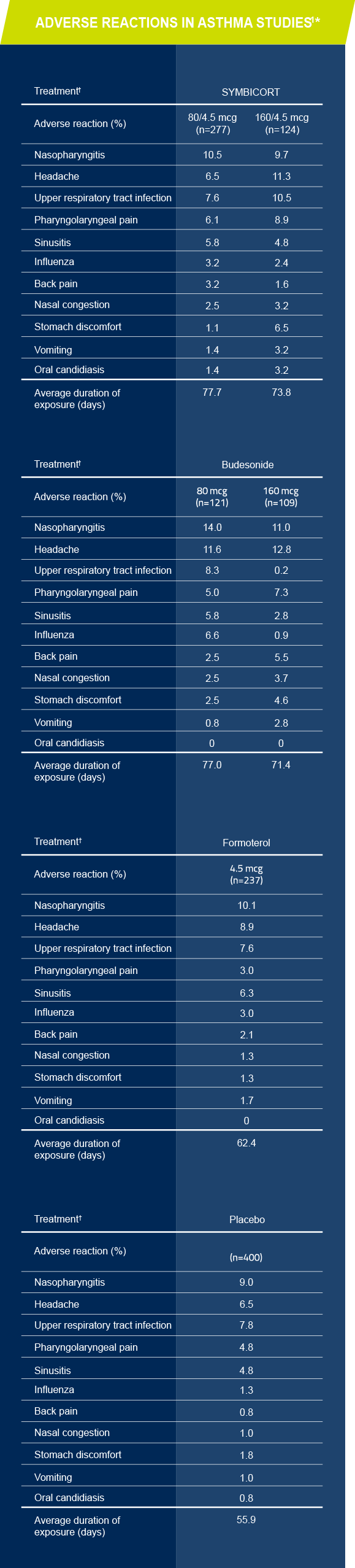
*The incidence of adverse reactions in this table is based upon pooled data from three 12-week, double-blind, placebo-controlled US asthma clinical trials in patients aged 12 years and older.
†All treatments were administered as 2 inhalations twice daily.
Please see full Prescribing Information ![]() , including Patient Information.
, including Patient Information.