How to Use the SYMBICORT Inhaler
Get information on how to use the SYMBICORT inhaler.
WATCH THE VIDEO
The majority of patients’ FEV1 improvement occurred at 15 minutes 1,2
In pediatric patients taking SYMBICORT 80/4.5† (n=90) in CHASE 3, 74% of 1-hour postdose FEV1 improvement occurred at 15 minutes on day of randomization and 86% at Week 122
Significant improvement in lung function was demonstrated in a 12-week efficacy and safety study of pediatric patients with asthma 6 to <12 years of age1,3
†Administered as 2 inhalations twice daily.
SYMBICORT is NOT a rescue medication and does NOT replace fast-acting inhalers to treat acute symptoms
The most common adverse reactions ≥3% reported in CHASE 3 in pediatric patients with asthma treated with SYMBICORT 80/4.5 included upper respiratory tract infection, pharyngitis, headache, and rhinitis.
Significant improvement in FEV1 at 12 weeks1,2
Change from baseline in 1-hour postdose FEV1 over 12 weeks3
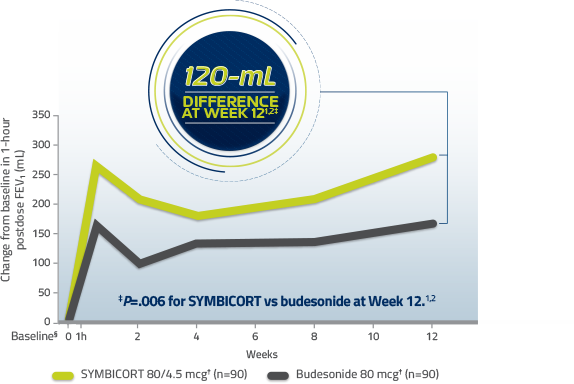
Data from an efficacy and safety study of pediatric asthma patients 6 to <12 years of age
SYMBICORT is NOT a rescue medication and does NOT replace fast-acting inhalers to treat acute symptoms
†Administered as 2 inhalations twice daily.
§Baseline is defined as the predose FEV1 value on day of randomization (Week 0).1
STUDY DESIGN: CHASE 3
CHASE 3: A 12-week, randomized, double-blind, multicenter study of 184 pediatric patients 6 to less than 12 years of age with a documented clinical diagnosis of asthma and on daily medium-dose range ICS or fixed combination of ICS and LABA therapy compared SYMBICORT pMDI 80/4.5 mcg (n=92) with budesonide pMDI 80 mcg (n=92), each administered as 2 inhalations twice daily. The study included a 2- to 4-week run-in period with a low-dose ICS followed by a 12-week treatment period. This study was designed to assess change from baseline to Week 12 in 1-hour postdose FEV1 (primary endpoint). Secondary efficacy variables included predose and 1-hour postdose PEF, predose and 15-minute postdose FEV1, nighttime awakenings, rescue albuterol use, and Pediatric Asthma Quality of Life Questionnaire scores.
SYMBICORT 80/4.5 FOR PEDIATRIC ASTHMA PATIENTS 6 to YEARS
| FOR PEDIATRIC PATIENTS 6 TO LESS THAN 12 YEARS OF AGE3 | |
|---|---|
| 80/4.5 mcg | 2 inhalations BID|| |
SYMBICORT is NOT a rescue medication and does NOT replace fast-acting inhalers to treat acute symptoms

CONSIDER SYMBICORT 80/4.5 FOR YOUR APPROPRIATE PEDIATRIC ASTHMA PATIENTS AGED 6 to YEARS
||Administered in the morning and evening.
¶Pressurized metered-dose inhaler.
#The actual amount of drug delivered to the lung may depend on patient factors, such as the coordination between actuation of the device and inspiration through the delivery system.
**Dry powder inhaler.
Please see full Prescribing Information ![]() , including Patient Information.
, including Patient Information.